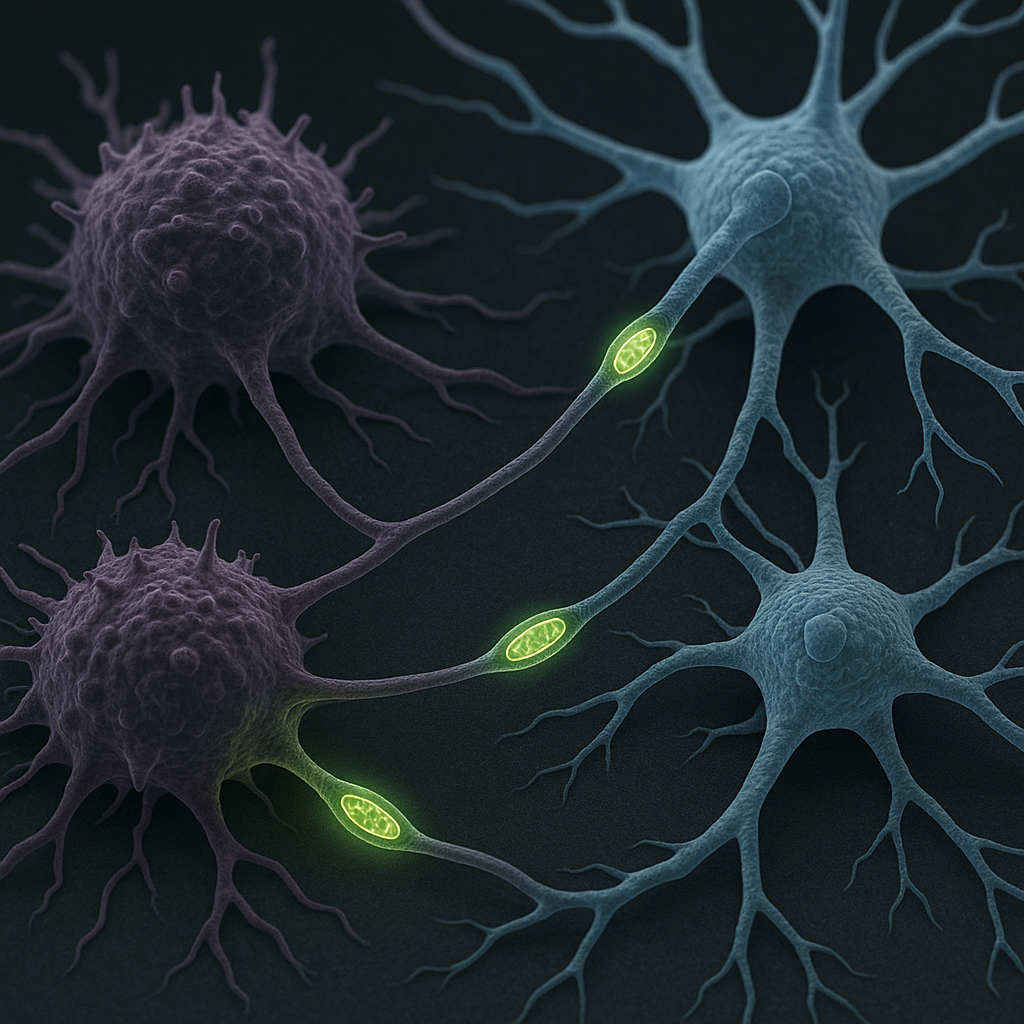
Mechanism of Mitochondrial Hijacking Discovered
Cancer cells have developed a sophisticated mechanism to enhance their survival during metastasis by stealing mitochondria—the cellular powerhouses—from nearby nerve cells. This process, termed ‘energy theft,’ involves the formation of microscopic tubular connections that allow cancer cells to siphon mitochondria directly from nerve cells, significantly boosting their metabolic resilience.
Table of Contents
Cellular Mechanism and Process
The hijacking process begins when cancer cells extend thin, tube-like protrusions toward adjacent nerve cells. These microscopic conduits, measuring approximately 50-200 nanometers in diameter, create direct cytoplasmic connections between the two cell types. Once established, these tubes facilitate the unidirectional transfer of mitochondria from nerve cells to cancer cells.
Metabolic Advantages for Cancer Cells
- Enhanced ATP production capacity through additional mitochondria
- Improved resistance to metabolic stress during metastatic spread
- Increased cellular energy reserves for invasion and proliferation
- Better adaptation to oxygen-limited environments
Implications for Anti-Metastasis Therapy
This discovery identifies mitochondrial hijacking as a potential therapeutic target for preventing cancer metastasis. Blocking the formation of these intercellular tubes or disrupting the mitochondrial transfer process could significantly reduce cancer cells’ ability to survive and spread throughout the body. Current research focuses on identifying specific proteins involved in tube formation and mitochondrial trafficking.
Research Methodology and Findings
Researchers utilized advanced live-cell imaging techniques and fluorescent mitochondrial markers to observe the real-time transfer of mitochondria between cancer and nerve cells. The study involved multiple cancer cell lines and primary nerve cell cultures, with consistent observations across different experimental conditions.
Stay Updated
Join our newsletter for the latest updates and exclusive content.